malulu
NJRC Member
I have a simple question, may be some of you know the answer to?

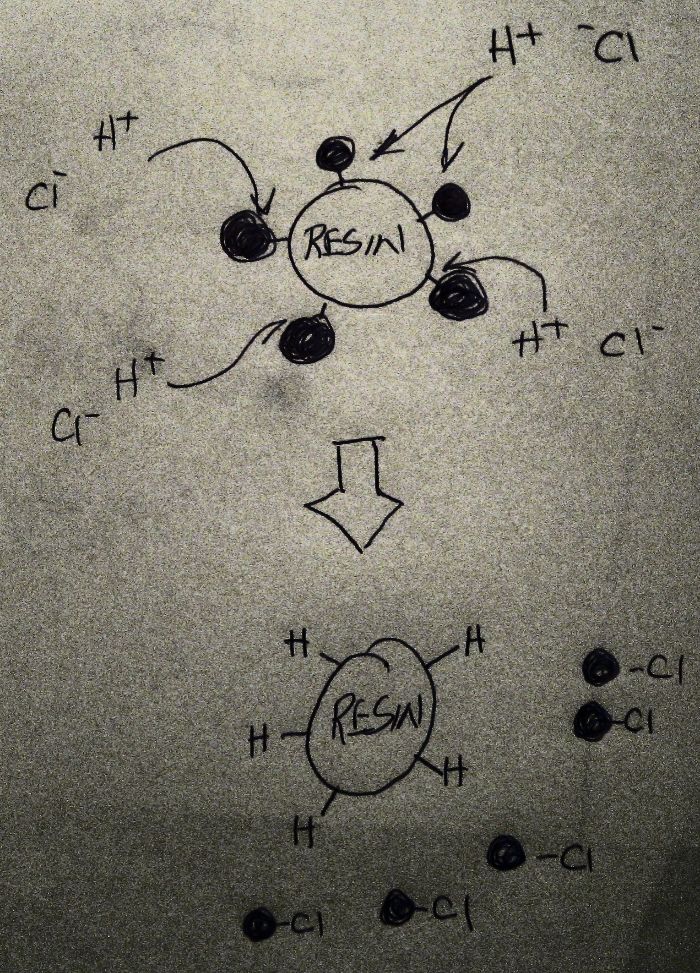
I have a Kati-Ani unit (to make pure water like RO/DI)...
it comes with 2 Medias....( KATI & ANI )
to recharge - it required to use Muriatic-Acid on the KATI unit, and NaOH on the ANI unit.
when mixing the solution to run through the unit...
it said 1:6 (Acid:Water) run through the KATI unit for about 45+ mins
for my unit #5, it need about 4.5 gals of stuff (can be easily using a regular 5-gals bucket for this task)
QUESTION is:
- i have another set of unit #5 which also need to recharge, due to i don't have a bigger containers...
- can i use the same 5-gals bucket to double it up as 2:6 (Acid:Water) and then run through the unit with 90+ mins (slower drip rate...)
- would it be the same result? or i need to do the same process twice as the dosage as the instruction suggested?
- what about the ANI unit? can i applied the same shortcut to the use stronger NaOH with same amount of water and slower drip rate/
thanks in advance for the great advises to come...

NOTE:
the whole recharging process can be found here:
http://www.njreefers.org/showthread.php?71200-Re-Charging-a-Cati-Ani-unit&highlight=kati-ani
I have a Kati-Ani unit (to make pure water like RO/DI)...
it comes with 2 Medias....( KATI & ANI )
to recharge - it required to use Muriatic-Acid on the KATI unit, and NaOH on the ANI unit.
when mixing the solution to run through the unit...
it said 1:6 (Acid:Water) run through the KATI unit for about 45+ mins
for my unit #5, it need about 4.5 gals of stuff (can be easily using a regular 5-gals bucket for this task)
QUESTION is:
- i have another set of unit #5 which also need to recharge, due to i don't have a bigger containers...
- can i use the same 5-gals bucket to double it up as 2:6 (Acid:Water) and then run through the unit with 90+ mins (slower drip rate...)
- would it be the same result? or i need to do the same process twice as the dosage as the instruction suggested?
- what about the ANI unit? can i applied the same shortcut to the use stronger NaOH with same amount of water and slower drip rate/
thanks in advance for the great advises to come...
NOTE:
the whole recharging process can be found here:
http://www.njreefers.org/showthread.php?71200-Re-Charging-a-Cati-Ani-unit&highlight=kati-ani